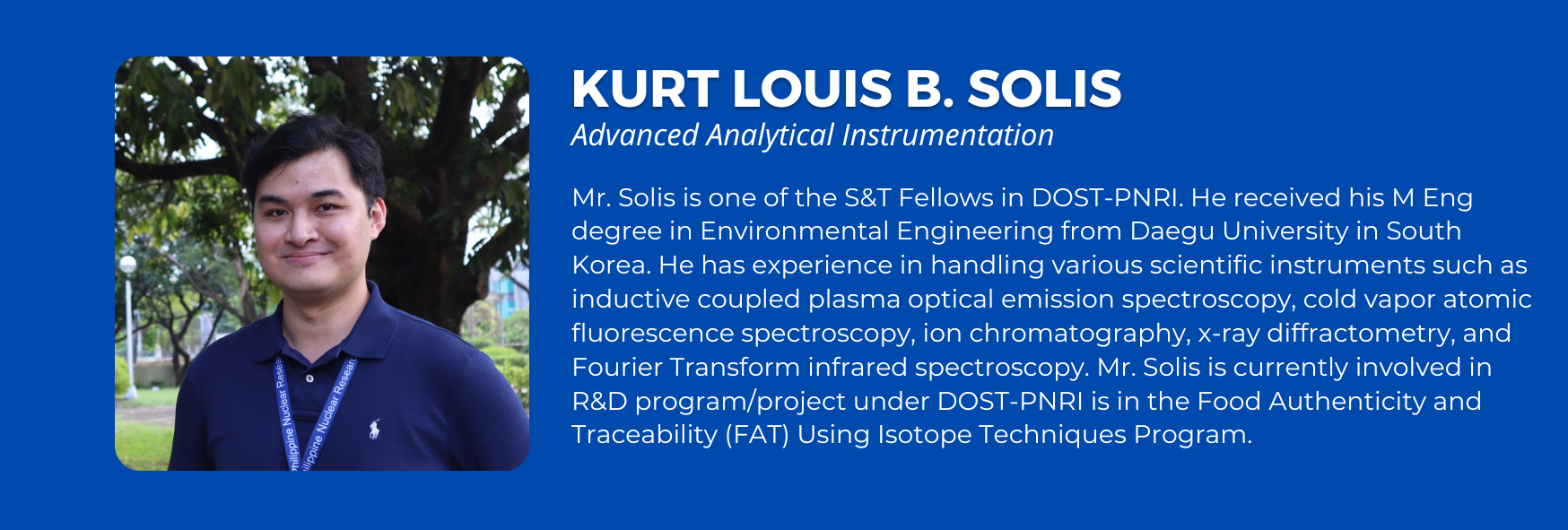
Sex: Male
Education:
- Master of Engineering in Environmental Engineering, Daegu University South Korea, 2017
- Bachelor of Science in Chemistry, University of the Philippines Diliman, 2014
Field of Specialization:
Environmental chemistry
Researches:
Article title: Reduction in mercury bioavailability to Asian clams (Corbicula fluminea) and changes in bacterial communities in sediments with activated carbon amendment
Authors: Mark Xavier Bailon, Minoh Park, Kurt Louis Solis, Yeong Na, Dhiraj Kumar Chaudhary, Sungpyo Kim, Yongseok Hong
Publication title: Chemosphere 291(1): 132700, 2021
Abstract:
Activated carbon (AC) amendment is considered as one of the alternatives for managing and remediating mercury (Hg) contaminated sediments because of its high sorptive capacity and potential to immobilize the contaminant. For this study, the underlying mechanisms that control the reduction of Hg bioavailability in AC-amended estuarine sediments were investigated in box microcosm set-ups with 28-day Asian clam bioassay experiments. The application of diffusive gradients in thin film technique (DGT) revealed that the total mercury and methylmercury levels in sediment pore water decreased by 60%–75% in 1%–3% AC-amended sediments. This decrease subsequently led to a linear reduction in the Hg body burden in Asian clams, even at 1% sorbent mixing. These observations implied that AC amendment reduced the net flux of Hg into the pore water and overlying water, resulting in reduced Hg bioaccumulation in benthic organisms. The addition of AC to sediment also led to reduced dissolved organic carbon and several biogeochemical indicators (HS−, Mn, and Fe) in the pore water. Furthermore, the 16 S rRNA gene amplicon sequencing analysis revealed noticeable alterations in the microbial communities after AC amendment. The predominant phylum was Firmicutes in control sediment, Bacteroidetes in 1% AC-amended sediment, and Proteobacteria in both 2% and 3% AC-amended sediment samples. The genera-level analysis showed that the relative abundance of the Hg-methylators decreased as the level of AC amendment increased. These observations suggested that AC amendment decreased Hg bioavailability not only by physicochemical sorption but also by changing geochemical species and shifting the microbial community composition.
Full text link available upon request to the author
Article title: Sustainable removal of Hg(II) by sulfur-modified pine-needle biochar
Authors: Cheolho Jeon, Kurt Louis Solis, Ha-Rim An, Yongseok Hong, Avanthi Deshani, Igalavithana, Yong Sik Ok
Publication title: Journal of Hazardous Materials 388: 122048
Abstract:
Sulfur-modified pine-needle biochar (BC–S) was produced for the removal of Hg(II) in aqueous media via post-pyrolysis S stream exposure. Fourier-transform infrared spectroscopy, elemental analysis, and X-ray photoelectron spectroscopy confirmed the addition of S(0) groups on the surface of BC–S. Hg(II) adsorption on BC–S was best described by the Freundlich isotherm with a KF of 21.0 mg L g−1 and a pseudo-second-order adsorption kinetics model with a rate of 0.35 g mg−1 min−1. Hg(II) removal on BC–S was found to be an endothermic process that relied on C-Hg and S-Hg interactions rather than reduction by S(0) groups. The adsorption increased with increasing solution pH and decreased with increasing dissolved organic matter concentration, but was unaffected by increasing salt concentrations. BC–S showed a maximum of 3 % S leaching in aqueous media after 28-d exposure time, and exposure to aqueous media did not convert Hg(II) to elemental Hg. Overall, BC–S exhibited superior Hg(II) removal performance over unmodified BC, thus having potential applications in natural water and wastewater treatment with no significant threat of secondary pollution.
Full text link available upon request to the author
Article title: Metal Organic Framework UIO-66 and activated carbon composite sorbent for the concurrent adsorption of cationic and anionic metals.
Authors: Kurt Louis B. Solis, Young-Hwan Kwon, Moon-Hyeon Kim, Ha-Rim An, Cheolho Jeon, Yongseok Hong
Publication title: Chemosphere 238: 124656, January 2020
Abstract:
A composite sorbent for the simultaneous removal of both Hg2+ and SeO32− from aqueous media was produced from the solvothermal synthesis of a zirconium metal organic framework, UiO-66, in the presence of activated carbon. The composite sorbent has a large surface area of 1051 m2 g−1 with crystalized porous structures and has strong thermal stability up to 600 °C. The contaminant uptake of the sorbent follows a Langmuir adsorption isotherm with maximum sorption capacity of 205 mg g−1 and 168 mg g−1 for Hg2+ and SeO32−, respectively. Scanning electron microscopy-energy dispersive spectroscopy results show that the Se regions overlap exclusively with Zr-rich regions suggesting that SeO32− adsorption depends entirely on the exposed UiO-66 surface. In addition, X-ray photoelectron spectroscopy spectra of Se 3d and Hg 4f showed the association of SeO32− and Hg2+ on the UiO-66 and carbon surfaces, respectively. The sorbent could facilitate the development of a single process for the simultaneous removal of cationic Hg and anionic Se as well as other similar ionic metals with opposite charges from aqueous media.
Full text link available upon request to the author
Article title: Spatiotemporal variation of groundwater arsenic in Pampanga, Philippines.
Authors: Kurt Louis B. Solis,Reygie Q. Macasieb,Roel C. Parangat, Jr., Augustus C. Resurreccion, Joey D. Ocon
Publication title: Water 12(9):2366, 2020
Abstract:
Several confirmed cases of arsenic (As) poisoning have been reported in Central Luzon, the Philippines, in recent years. There is a growing interest in As research in the Philippines due to the reported As poisoning cases. However, an extensive spatiotemporal As study has not been conducted. In this work, As concentration measurements were conducted in 101 wells in Guagua, Pampanga, in Central Luzon, the Philippines, from November 2018 to November 2019. The wells included 86 public hand pumps, 10 pumping stations, and 5 private, jet-powered pumps. Using hydride generation—inductively coupled plasma—optical emission spectroscopy (HG-ICP-OES), analysis of the wells in 12 barangays in Guagua revealed that 38.7% had average As concentrations beyond the 10 ppb limit with some wells having high Mn (4.0 ppm) and Fe (2.0 ppm) content as well. The high pH and reducing conditions in the wells in Guagua may have contributed to the persistence of As in the groundwater. The mean difference in wet season versus dry season As measurements were −4.4 (As < 10 ppb), −13.2 (10 to 50 ppb As), and −27.4 (As > 50 ppb). Eighty-three wells (82.2%) had higher As concentrations in the dry season, 8 wells (7.92%) had higher As concentrations in the wet season, 7 wells (6.93%) had no significant difference between the wet and dry season, and 3 wells had been decommissioned. These results indicate that there is a significant difference in As concentrations in the wet and dry seasons, and this could have implications in water treatment technology and policy implementation. The work resulted in the first year-long characterization of groundwater As in the Philippines.
Full text link https://tinyurl.com/mrx3ea7b
Article title: Effectiveness of gold nanoparticle-coated silica in the removal of Inorganic Mercury in aqueous systems: Equilibrium and Kinetic Studies.
Authors: Kurt Louis Solis, Go-Un Nam, Yongseok Hong
Publication title: Environmental Engineering Research, 21(1), 99–107, 2016
Abstract:
The adsorption of inorganic mercury, Hg (II), in aqueous solution has been investigated to evaluate the effectiveness of synthesized gold (Au) nanoparticle-coated silica as sorbent in comparison with activated carbon and Au-coated sand. The synthesis of the Au-coated silica was confirmed by x-ray diffraction (Bragg reflections at 38.2°, 44.4°, 64.6°, and 77.5°) and the Au loading on silica surface was 6.91±1.14 mg/g. The synthesized Au-coated silica performed an average Hg adsorption efficiency of ~96 (±2.61) % with KD value of 9.96 (±0.32) L/g. The adsorption kinetics of Hg(II) on to Au-coated silica closely follows a pseudo-second order reaction where it is found out to have an initial adsorption rate of 4.73 g/μg/min/ and overall rate constant of 4.73 × 10−4 g/μg/min/. Au-coated silica particles are effective in removing Hg (II) in aqueous solutions due to their relatively high KD values, rapid adsorption rate, and high overall efficiency that can even decrease mercury levels below the recommended concentrations in drinking water.
Full text link https://tinyurl.com/ymcdzkc2
Article title: Mercury(II) reduction and sulfite oxidation in aqueous systems: kinetics study and speciation modeling
Authors: Kurt L. B. Solis A , Go-un Nam A and Yongseok Hong
Publication title: Environmental Chemistry, 14(3), 151, 2017
Abstract:
Environmental contextWastewater contains various substances such as sulfur-containing chemicals and heavy metals including mercury ions. Several technologies have been developed to trap mercury ions; however, mercury can undergo reactions with sulfite and change to its vapour form, which easily escapes to the atmosphere. Here, we devised a model to predict the formation of vapour-phase mercury as a function of sulfite concentration, temperature and water acidity based on coal-fired power plant wastewater. AbstractThe re-emission of mercury (Hg) as a consequence of the formation and dissociation of the unstable complex HgSO3 is a problem encountered in flue gas desulfurisation treatment in coal-fired power plants. A model following a pseudo-second-order rate law for Hg2+ reduction was derived as a function of [SO32-], [H+] and temperature and fitted to experimentally obtained data to generate kinetics rate values of 0.120±0.04, 0.847±0.07, 1.35±0.4mM-1 for 40°C, 60°C and 75°C respectively. The rate of reduction of Hg2+ increases with a temperature increase but shows an inverse relationship with proton concentration. Plotting the model-fit kinetics rate constants yields ΔH≤61.7±1.82 kJ mol-1, which is in good agreement with literature values for the formation of Hg0 by SO32-. The model could be used to better understand the overall Hg2+ re-emission due to SO32-happening in aquatic systems such as flue gas desulfurisation wastewaters.
Full text link available upon request to the author
Article title: KOH activated pine tree needle leaves biochar as effective sorbent for VOCs in water.
Authors: Nshirirungu Theoneste, Moon Hyun Kim, Kurt Louis Solis, Minoh Park and Yongseok Hong
Publication title: Membrane Water Treatment, 9(5), 293-300, 2018
Abstract:
The removal of volatile organic compounds (VOCs) from water using KOH-activated pine tree needle leaves biochar is considered a cost effective and efficient process. In this study, pine tree needle leaves were mixed with 0, 50, 100 and 200% (KOH weight/feedstock weight) of KOH, respectively. Then, the mixture was pyrolyzed at 500 degree celcius for 6 hrs. The adsorption characteristics of 10 VOCs to the biochar were tested. The results indicated that the removal efficiency of the KOH activated biochar was highest in 100% KOH-biochar. The VOC removal efficiencies of 50% and 200% KOH activated biochar were similar and the 0% KOH activated biochar showed the lowest VOC removal. The FTIR results showed that increasing the amount of KOH seemed to enhance the formation of various functional groups, such as -OH, -C=C, -O. The adsorption strength of 10 VOCs to the KOH activated biochar seemed to be increasing by the increase of the solubility of VOCs. This may suggest that the adsorption is taking place in hydrophilic sites of the biochar surface. The KOH activated pine tree needle leaves biochar can be an effective sorbent for VOCs removal in water and 100% KOH mixing seemed to provide better sorption capacity.
Full text link available upon request to the author
Contact details: This email address is being protected from spambots. You need JavaScript enabled to view it.